We manufacture three sizes of silver/silver chloride/0.5M KCL Reference Electrodes to manage cathodic protection systems in reinforced concrete or steel framed buildings. Please note that these electrodes are not suitable for submerged seawater or splash zones where we recommend using our RB15 manganese dioxide reference electrode.
Please take your pick from the selection below to find your required reference electrode based on your required service life and reference electrode size.
Data sheets and installation procedures for the electrodes can be found in the panel on the right of this page. If you order the units without cable tails please follow our instructions to connect your own cable tails.

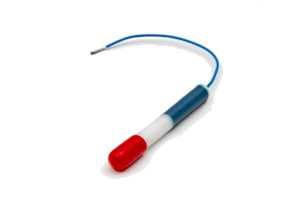
The LD10 is a long life silver/silver chloride/0.5M KCL reference electrode for permanent installation in atmospherically exposed concrete structures. It is not suitable for submerged seawater or splash zones where we recommend using our RB15 manganese dioxide reference electrode. The essential components are silver metal, silver chloride, soluble silver ions and chloride ions.
Ag, AgC1(s), C1-, Ag+
A sparingly soluble salt, silver chloride, is in equilibrium with a saturated solution of this salt which precipitates in the course of electrolysis. The reversible electrode reaction consists of silver ions going into solution and then combining with the chloride ions to form silver chloride. Thus its potential is determined by the following reactions:
Ag ↔ Ag+ e- Ag+ + Cl- ↔ AgC1(s) Ag+ C1- ↔ AgC1(s) + e-
The potential is dependent on temperature and the concentration of chloride ions in accordance with the following equation:
|
E=E0- |
RT |
1n[C1-] |
|
F |
Where E0, R, F and T are the standard potential, gas constant, Faraday Constant and temperature respectively. The reaction has been proved to obey these equations in solutions with pH's of between 0 and 13.5. The potential is however very sensitive to traces of bromide ions which make it more negative.
The electrode element has been prepared by electrolytic precipitation of silver chloride onto silver metal. This has then been embedded in a mortar containing a known concentration of chloride ions and an anti-drying agent. The housing consists of a white nylatron barrel and inserts
Element Type:
Silver/silver chloride
Typical 1.2g pure silver
Potential:
-15mV wrt saturated calomel electrode (SCE) at 200C
Drift:
+/-3mV in 24 hours
Typically less than +/- 10mV expected in 20 years
Dimensions:
80mm long x 10mm diameter
Housing:
White nylatron barrel and inserts
Cable:
Raychem cable product code 44A0111-16-6
Expected Life:
More than 30 years at a leakage current of 1µA will result in the loss of 0.7 grams of silver. The functional life of the electrode will most likely to be determined by the life of the associated cables.
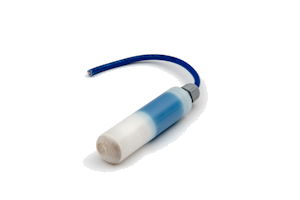
The LD15 is a long life silver/silver chloride/0.5M KCL reference electrode for permanent installation in atmospherically exposed concrete structures. It is not suitable for submerged seawater or splash zones where we recommend using our RB15 manganese dioxide reference electrode. The essential components are silver metal, silver chloride, soluble silver ions and chloride ions.
Ag, AgC1(s), C1-, Ag+
A sparingly soluble salt, silver chloride, is in equilibrium with a saturated solution of this salt which precipitates in the course of electrolysis. The reversible electrode reaction consists of silver ions going into solution and then combining with the chloride ions to form silver chloride. Thus its potential is determined by the following reactions:
Ag ↔ Ag+ e-Ag+ + Cl- ↔ AgC1(s)Ag+ C1- ↔ AgC1(s) + e-
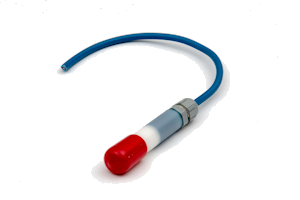
The potential is dependent on temperature and the concentration of chloride ions in accordance with the following equation:
|
E=E0- |
RT |
1n[C1-] |
|
F |
Where E0, R, F and T are the standard potential, gas constant, Faraday Constant and temperature respectively. The reaction has been proved to obey these equations in solutions with pH's of between 0 and 13.5. The potential is however very sensitive to traces of bromide ions which make it more negative.
The electrode element has been prepared by electrolytic precipitation of silver chloride onto silver metal. This has then been embedded in a mortar containing a known concentration of chloride ions and an anti-drying agent. The housing consists of a white nylon barrel, white nylon inserts, and a cable gland rated at IP68.
Element Type:
Silver/silver chloride Typical 1.2g pure silver
Potential:
-15mV wrt saturated calomel electrode (SCE) at 200C
Drift:
+/-3mV in 24 hours
Typically less than +/- 10mV expected in 20 years
Dimensions:
85mm long x 15mm diameter
Housing:
White nylatron barrel and inserts with IP68 cable gland
Cable:
These electrodes can be supplied without cable for cable to be attached on site
Expected Life:
More than 30 years at a leakage current of 1µA will result in the loss of 0.7 grams of silver. The functional life of the electrode will most likely to be determined by the life of the associated cables.
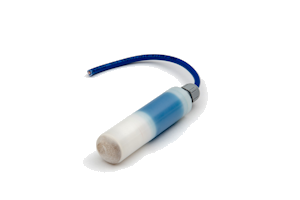
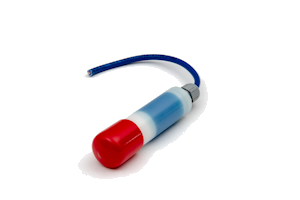
The LD25 is a long life silver/silver chloride/0.5M KCL reference electrode for permanent installation in atmospherically exposed concrete structures. It is not suitable for submerged seawater or splash zones where we recommend using our RB15 manganese dioxide reference electrode. The essential components are silver metal, silver chloride, soluble silver ions and chloride ions.
Ag, AgC1(s), C1-, Ag+
A sparingly soluble salt, silver chloride, is in equilibrium with a saturated solution of this salt which precipitates in the course of electrolysis. The reversible electrode reaction consists of silver ions going into solution and then combining with the chloride ions to form silver chloride. Thus its potential is determined by the following reactions:
Ag ↔ Ag+ e-Ag+ + Cl- ↔ AgC1(s)Ag+ C1- ↔ AgC1(s) + e-
The potential is dependent on temperature and the concentration of chloride ions in accordance with the following equation:
|
E=E0- |
RT |
1n[C1-] |
|
F |
Where E0, R, F and T are the standard potential, gas constant, Faraday Constant and temperature respectively. The reaction has been proved to obey these equations in solutions with pH's of between 0 and 13.5. The potential is however very sensitive to traces of bromide ions which make it more negative.
The electrode element has been prepared by electrolytic precipitation of silver chloride onto silver metal. This has then been embedded in a mortar containing a known concentration of chloride ions and an anti-drying agent. The housing consists of a white nylatron barrel, white nylatron inserts, and a cable gland rated at IP68.
Element Type:
Silver/silver chloride
Typical 3g pure silver
Potential:
-15mV wrt saturated calomel electrode (SCE) at 200C
Drift:
+/-3mV in 24 hours
Typically less than +/- 10mV expected in 20 years
Dimensions:
75mm long x 15mm diameter
Housing:
White nylatron barrel and inserts with IP68 cable gland
Cable:
These electrodes can be supplied without cable for cable to be attached on site.
Expected Life:
More than 50 years at a leakage current of 1µA will result in the loss of 1.0 grams of silver. The functional life of the electrode will most likely to be determined by the life of the associated cables.